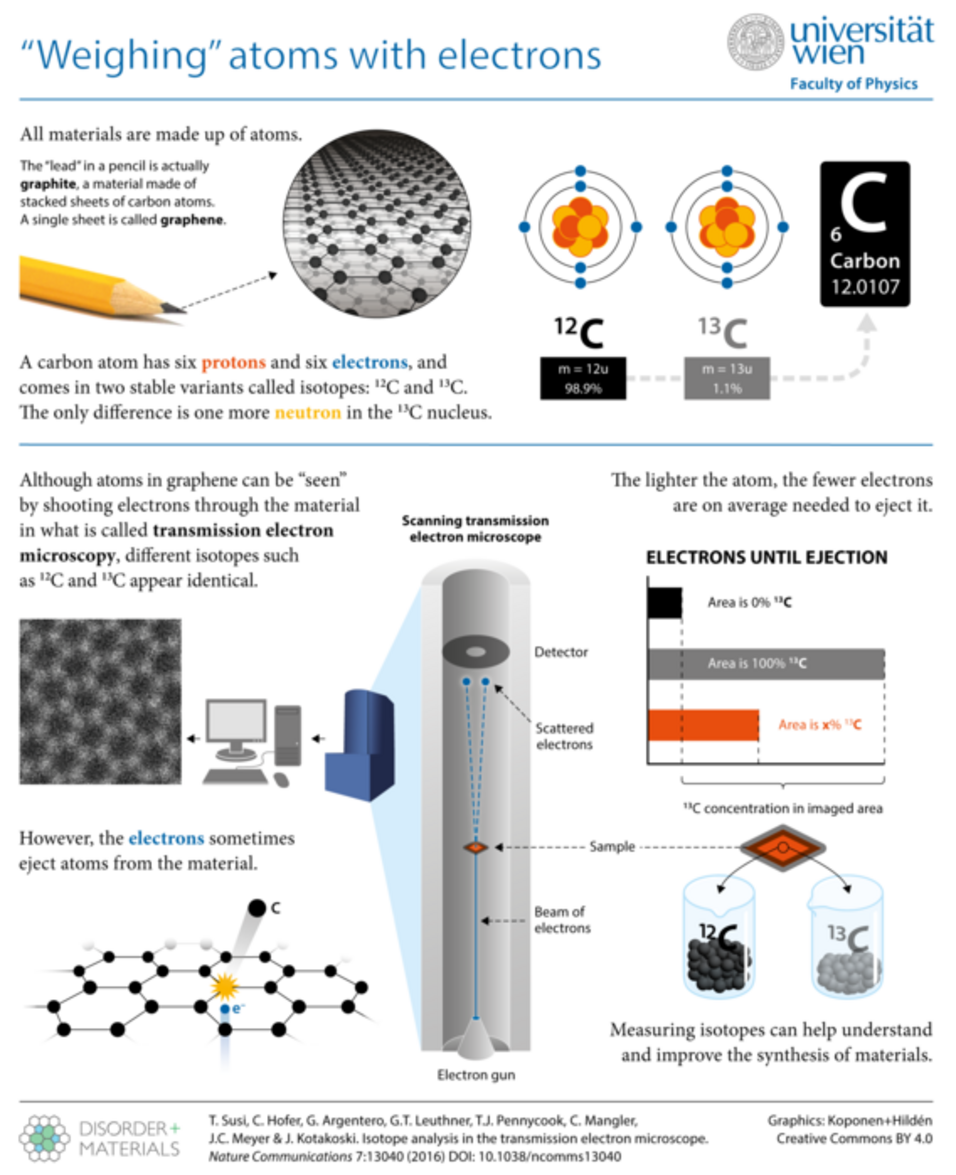
In our recent work, published in Nature Communications, we report a new way for "weighing” atoms by atomic-resolution imaging of graphene, the one-atom-thick sheet of carbon.
University press release: Weighing atoms with electrons
Publication: Isotope analysis in the transmission electron microscope: Toma Susi, Christoph Hofer, Giacomo Argentero, Gregor T. Leuthner, Timothy J. Pennycook, Clemens Mangler, Jannik C. Meyer & Jani Kotakoski. Nature Communications | 7:13040 | DOI: 10.1038/ncomms13040.
Open data: Atomic resolution electron irradiation time series of isotopically labeled monolayer graphene: Toma Susi, Christoph Hofer, Giacomo Argentero, Gregor T. Leuthner, Timothy J. Pennycook, Clemens Mangler, Jannik C. Meyer & Jani Kotakoski. figshare (2016). DOI: 10.6084/m9.figshare.c.3311946.v1.